
Regulatory Affairs Services – RX Trading
Ensuring Compliance, Accelerating Access
At RX Trading, our Regulatory Affairs Services play a critical role in navigating the complex global pharmaceutical landscape. With in-depth knowledge of international regulatory frameworks and a commitment to quality and compliance, we help our clients bring safe, effective, and compliant pharmaceutical products to market efficiently.
What We Offer
Our Regulatory Affairs team serves as the vital link between pharmaceutical innovation and government health authorities. We manage the entire lifecycle of regulatory processes, from product development to post-marketing compliance.

Our Key Regulatory Services Include:

Regulatory Strategy Development
We build customized regulatory roadmaps to support your product’s journey from concept to commercialization, ensuring minimal delays and optimized approval timelines.

Dossier Compilation & Submission
We prepare and submit high-quality regulatory dossiers (CTD/eCTD) for: New Drug Applications (NDAs) Marketing Authorization Applications (MAAs) Generic Submissions Over-the-Counter (OTC) Products Biologicals & Biosimilars

Product Registration
We manage end-to-end product registration processes across the UAE, GCC, and international markets, ensuring local compliance and timely approvals.

Regulatory Liaison
Our team coordinates directly with health authorities such as: UAE Ministry of Health and Prevention (MOHAP) GCC Drug Regulatory Authorities EMA, FDA, and other global regulatory bodies
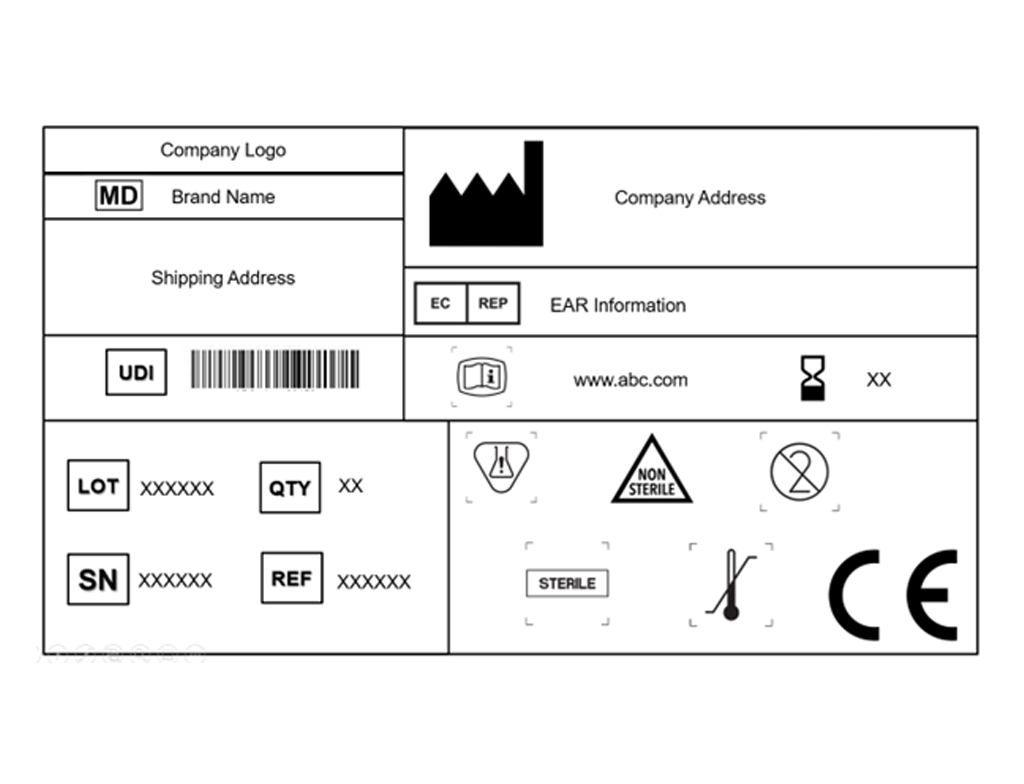
Labeling & Artwork Compliance
We ensure your product labels, leaflets, and packaging meet regulatory requirements and are up to date with current guidelines.
Post-Marketing Surveillance & Variations
From handling safety updates to managing variations and renewals, we support the long-term regulatory maintenance of your pharmaceutical products.
Why Choose RX Trading?
- Deep Local Expertise: We have extensive experience with UAE and GCC regulatory environments.
- Global Insight: Familiar with international markets including EMA, FDA, and WHO regions.
- Fast Turnaround: We help accelerate product approvals and market access.
- Accuracy & Compliance: Our meticulous approach ensures all submissions meet regulatory standards.
- Client-Focused: We provide tailored solutions and ongoing support at every stage of the product lifecycle.

Partner with RX Trading for Regulatory Excellence
Whether you’re launching a new product, expanding into new markets, or ensuring ongoing compliance, RX Trading is your trusted regulatory affairs partner in the pharmaceutical sector.
